 नवीनतम
नवीनतम
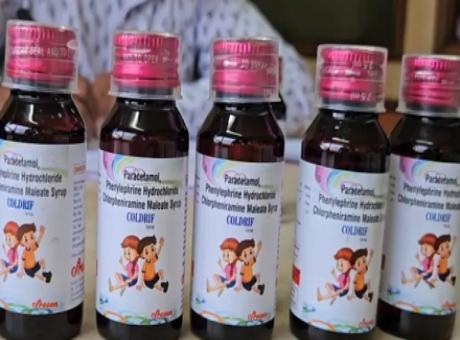
Cough Syrup deaths: Plea in SC seeks CBI probe
NEW DELHI [Maha Media]: A plea has been moved in the Supreme Court seeking an inquiry and systemic reform in drug safety mechanisms against the backdrop of deaths of children in Madhya Pradesh and Rajasthan, allegedly due to the consumption of toxic cough syrups.
Filed by advocate Vishal Tiwari, the plea sought a court-monitored probe into the incidents and also a direction for the constitution of a National Judicial Commission or Expert Committee headed by a retired Supreme Court judge.
The plea contended that a probe under the supervision of a retired Supreme Court judge would ensure fairness and uniformity. The petition submitted that separate probes at the state level have led to fragmented accountability, which has led to repeated lapses that allow hazardous formulations to reach the market.
It also urged the apex court that all pending FIRs and investigations concerning the deaths of children caused by toxic cough syrups across states should be transferred to the CBI. The plea sought the court's direction to the Centre to constitute a national-level judicial or expert body to identify the regulatory failures that allowed substandard medicines to reach the market.
The plea has also sought a direction from the court to mandate toxicological testing of all suspect products through NABL-accredited laboratories before any further sale or export is permitted. The petition has been filed amid reports from Madhya Pradesh and Rajasthan, where several children allegedly died after consuming a particular kind of cough syrup.
Meanwhile, the National Human Rights Commission on Monday issued notices to the Madhya Pradesh, Rajasthan, and Uttar Pradesh governments, directing them to probe allegations of children's deaths due to contaminated cough syrup and immediately ban the sale of spurious medicines.
It has also directed the Union Health Ministry, the Drugs Controller General of India (DCGI), the Central Drugs Standard Control Organisation (CDSCO), and the Directorate General of Health Services (DGHS) to order an investigation into the supply of spurious drugs and instruct all regional labs in states to collect the samples of spurious drugs and submit test reports.